
David Stepp - Lab

Professor
Leon Henri Charbonnier Endowed Chair in Physiology
envelope icon dstepp@augusta.edu
Office: CB-3211B
Office phone icon 706-721-1949
Lab: CB-3319
Lab phone icon 706-721-6761
id-card-alt icon FACULTY PROFILE - David Stepp
Jump to: Research Projects Lab Personnel
Research
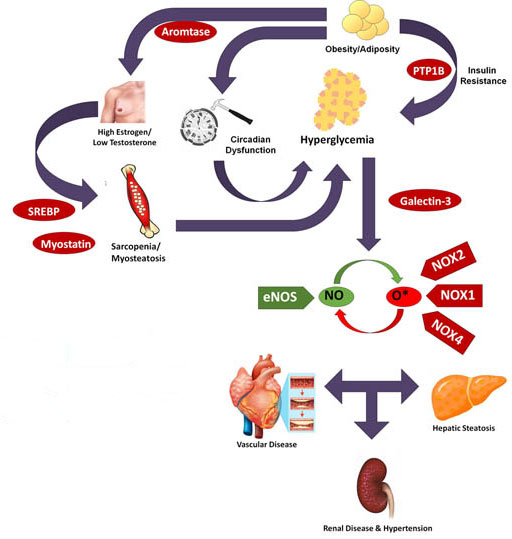
Work in our lab is devoted to unraveling links between obesity and cardiometabolic dysfunction. While weight loss is ideal, the overwhelming majority of the obese population – either due to chronic entrainment of metabolism or behavior, increased age or accumulation of injuries – retain or regain excess weight.
A critical need therefore is to break the links between obesity and its cardiovascular
outcomes. The CORE HYPOTHESIS of our laboratory is that resolution of endocrine and
metabolic abnormalities in obesity is the path to preventing cardiovascular disease
in overweight patients.
Currently, the central theme of these studies is that the balance of nitric oxide
(NO) derived from eNOS and the loss of its bioavailability from NADPH Oxidase (NOX)-generated
super-oxide is a key determinant of cardiometabolic health. Changes in glycemic status,
either absolute levels or pattern of hyperglycemia, appear to be a primary determinant
of states in which super-oxide excess reduces NO below a threshold needed for full
cardiovascular function. Thus, the glucose-NOS/NOX axis in the primary focus of studies
in our lab.
Projects
Disturbed circadian rhythm:
How glucose translates into cardiovascular dysfunction is a major source of inquiry in the field of cardiometabolic disease. Recent studies from our lab indicate that obese mice have a molecular cellular clock that has essentially run down, disconnecting normal diurnal signals from the anticipatory mechanisms that adapt vascular function to a changing environment. In parallel, obese mice show an inability to sudden changes in the circadian environment increasing glucose and NOX1, implying a role for the circadian clock in limiting the extent of obesity-related cardiovascular disease. The mechanisms of this process are the focus of this project.
Sex steroid imbalances:
Obese men commonly experience a drop in the male sex hormone testosterone and a rise in female hormones, notably estrogen. This imbalance in normal sex hormone levels is mediated by the enzyme aromatase which is expressed in fat and as fat expands, the pool of available aromatase expands in parallel. Obese men with high aromatase levels have an accelerated course of metabolic vascular disease independently of other risks present in these patients. The regulation of aromatase, it’s relation to body composition and its impact on the cardiovascular system in obese individuals is the focus of this project.
Ectopic lipid deposition:
A key aspect of the obese state is not just the accumulation of fat but the maldistribution of fat into tissues not normally involved in the storage of lipids – notably the liver and skeletal muscle. In addition to the derangement of local metabolism caused by lipid accumulation, it appears evidence that fat-laden muscle and liver cells cause additional metabolic imbalance that threaten the cardiovascular system. Provactively, it appears that alteration of the eNOS-NOX axis may be a contributing mechanism to ectopic lipid deposition, locking local metabolism into a downward spiral of excess lipids and poor perfusion. Breaking this spiral is the focus of this project.
Lab Personnel
James Mintz
Research Manager
jmintz@augusta.edu
Cody Bridgewater
PhD Candidate
cobridgewater@augusta.edu
Drew Speese
PhD Candidate
aspeese@augusta.edu