Nicholas R.J. Gascoigne, PhD, FRSB

Nicholas R.J. Gascoigne, PhD, FRSB
Professor, Immunology Center of Georgia
Lamar B. Peacock, M.D. Distinguished Chair for the Study of Immunological Disease
Professor, Department of Medicine, Medical College of Georgia, Augusta University
Member of Cancer Immunology, Inflammation, & Tolerance (CIIT), Georgia Cancer Center
Cellular immunotherapy using chimeric antigen receptor-bearing T cells (CAR-T) has made enormous advances in the treatment of liquid cancers over the last 10-15 years.
However, CAR-T are not very effective against solid tumors and the nature of the treatment, where T cells from the patient are removed, made to express the CAR through gene transfer, expanded in tissue culture, and then reinfused into the patient, is very time consuming and expensive. Many patients are unable to access the treatment either due to the costs or to the fact that they do not have sufficient healthy T cells to turn into CAR-T cells.
The Gascoigne lab recently discovered that CAR-T cells can be made more effective by using a fundamental difference in signal transduction between the natural T cell receptor (TCR) and the CAR. TCR signaling requires a protein tyrosine kinase called LCK for the T cells to become activated and kill tumor cells. Certain kinds of CAR that are currently in use in the clinic — very surprisingly — do not need LCK. Instead they can use another, related, kinase called FYN. Not only did knocking out LCK allow the CAR-T to be more specific and effective than regular CAR-T cells where LCK was active, but they even had stronger effectiveness against solid tumor (Wu et al. Cell Rep Med 4: 100917, 2023).
The fact that LCK-deficient CAR-T cells can function without the ability to be stimulated though the natural endogenous TCR means that they are suitable for allogeneic therapy. An allogeneic CAR-T would be able to be used as an off-the-shelf therapy, where a single healthy donor could provide therapy for hundreds of individual unrelated patients. The fact that TCR cannot signal means that they cannot cause graft versus host disease (GVHD). The lab is trying to develop this as a therapy. In addition, the lab has screened CRISPR libraries for novel genes that will enhance CAR-T function, and indeed, normal T cell function. A number of strong “hits” are being developed as potential therapeutic targets.
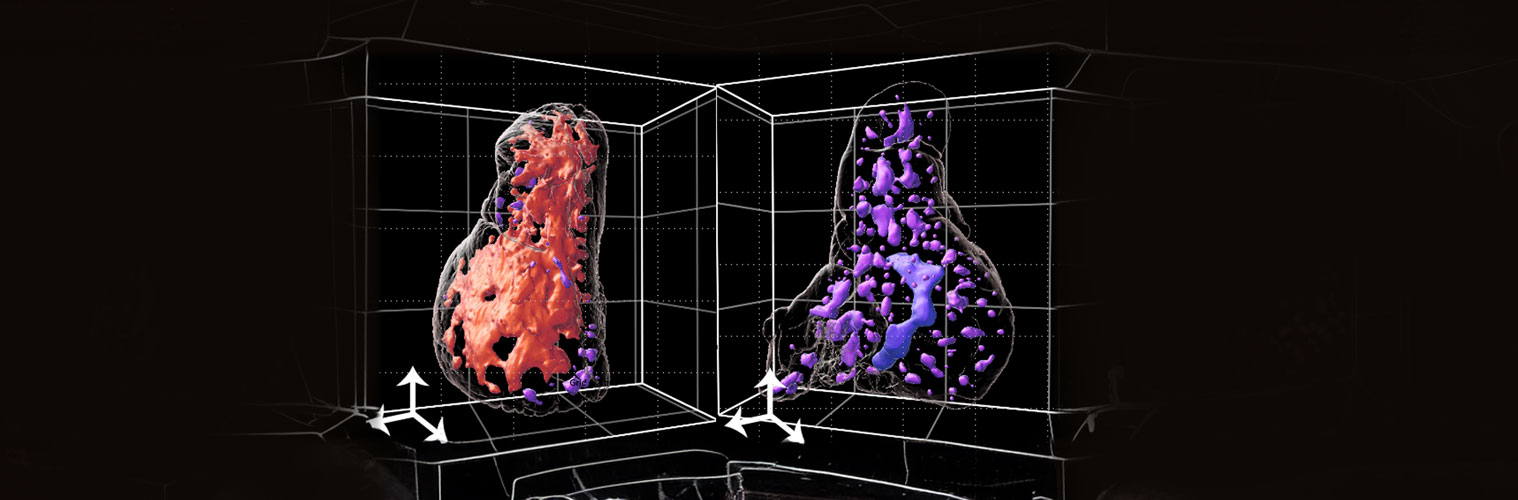
In addition, the lab identified and characterized Themis, a protein that controls thymocyte positive selection through regulation of TCR signaling strength. It also has an important role in coordinating TCR and cytokine signals in mature T cells. The lab is currently interested in the role of Themis in T cell signaling in the thymus and in formation of memory cells. Using 3D imaging of the thymus, the lab identified a single major lymphatic duct that appears to be a site of egress from the thymus, and that egress is not solely through blood vessels. How this works is under active study.
Research
-
The Gascoigne Lab focuses on T cell signaling, activation, and development, including signaling pathways in CAR-T cells and how they can be manipulated to improve CAR-T function in cancer immunotherapy.