- Augusta University
- Colleges & Schools
- Medical College of Georgia
- Physiology
- Faculty | Physiology
- Paul O'Connor, PhD
Paul O'Connor, PhD
Professor
Phon e: (706) 721-7890
e: (706) 721-7890
Fax: (706) 721-7299
Email: paoconnor@augusta.edu
Office: CB-2206
Lab: CB-2210
Learn more about Dr. O'Connor's Research
Education and Training
Monash University, Australia, B.Sc (Hons) 1997-2001 Biomedical Science
Monash University, Department of Physiology, Australia, PhD 2001-2005 Biomedical Sciences (Physiology)
Medical College of Wisconsin, Milwaukee, WI Post-doc 2005-2009 Physiology
Academic Appointments
July 2021 - present: Professor (Tenure), Department of Physiology, Medical College of Georgia, Augusta University
June 2016 - June 2021: Associate Professor (Tenure), Department of Physiology, Medical College of Georgia, Augusta University
January 2012 - June 2016: Assistant Professor (Tenure track), Department of Physiology, Medical College of Georgia, Augusta University
December 2011 - December 2012: Assistant Professor (Tenure track), Department of Medicine, Section of Experimental Medicine, Georgia Health Sciences Univeristy
2009 - November 2011: Assistant Professor (Research) Department of Physiology, Medical College of Wisconsin
Research Interests
Our laboratory’s interests lie in the physiological pathways involved in the regulation of kidney function and how disruptions in these pathways can lead to disease. In the laboratory, we utilize a number of approaches to study kidney function from the level of the awake animal down to cellular and molecular pathways. Dr. O’Connor’s early work focused on the mechanisms that regulate renal oxygen tension and are highlighted in Brenner and Rectors the Kidney 9th Edition.
Major research contributions from the O’Connor laboratory include:
1. Identification and characterization of the voltage-gated proton channel in the kidney including identification of mitochondrial derived reactive oxygen species as the source of Hv1 dependent reactive oxygen species in the kidney.
a. Voltage gated proton channels modulate mitochondrial reactive oxygen species production by complex I in renal medullary thick ascending limb. Redox Biology 2019
b. Hv1 acts as a sodium sensor and promotes superoxide production in medullary thick ascending limb of Dahl salt-sensitive rats. Hypertension 2014
2. Demonstrating the NaHCO3 ingestion promotes a systemic anti-inflammatory response in rats and healthy human subjects and that stimulation of gastric acid secretion may promote the cholinergic anti-inflammatory pathway.
a. Oral NaHCO3 activates the splenic anti-inflammatory pathway; evidence vagal signals are transmitted via mesothelial cells. Journal of Immunology 2018. *** Most downloaded article of 2018 in JI ***
b. A basic solution to activate the cholinergic anti-inflammatory pathway via the mesothelium? Pharmacologic Research 2019.
3. Demonstrating that vasa recta pericytes protect the kidney from vascular congestion following periods of ischemia.
a. Vasa recta pericyte density is negatively associated with vascular congestion in the renal medulla following ischemia reperfusion in rats, Am J Physiol 2017.
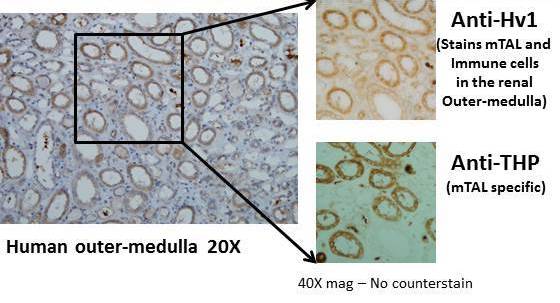
Voltage-gated proto channel (Hv1) expression in medullary thick ascending limb (mTAL) of the human kidney
Current Projects
Current projects in the O’Connor laboratory focus on three major themes:
1. Investigating the role of vasa recta in renal medullary vascular congestion. The renal medulla is susceptible to aggregation of RBC’s following periods of ischemia which can lead to prolonged hypoxia and cell death. The O’Connor laboratory is investigating methods to prevent vascular congestion and determine whether this can reduce long term renal injury as well as cardiovascular consequences following renal ischemia.
Renal vascular congestion is caused by a mismatch between cortical and medullary perfusion
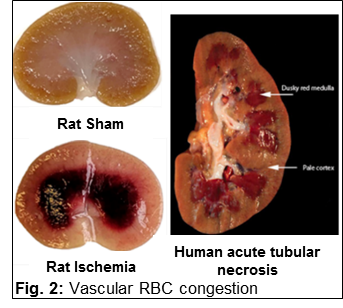
Vascular congestion (dark red) in the renal outer-medulla of the rat and human kidney following a period of ischemia
2. Determining the mechanisms through which NaHCO3 ingestion slows the decline in
renal function in CKD.A number of small clinical trials have demonstrated that oral ingestion of NaHCO3 markedly slows the decline in kidney function in CKD patients. The mechanisms underlying
this protective effect of NaHCO3 supplementation however, remain unclear. Our group has shown that ingestion of NaHCO3 promotes an anti-inflammatory polarization within the innate immune system, promoting
more regulatory or M2 macrophages and less classically activated or inflammatory M1
macrophages in the kidney. Our data indicate that this anti-inflammatory effect of
NaHCO3 ingestion requires the spleen and may be related to activation of the cholinergic
anti-inflammatory pathway, an innate anti-inflammatory pathway that can be promoted
by electrical stimulation of the vagal nerve. As inflammation may play an important
role in the progression of CKD, cost-effective and simple interventions that can modulate
the immune system and promote renal protection may be of great benefit to CKD patients.
O’Connor laboratory members are currently investigating how NaHCO3 ingestion promotes an anti-inflammatory response in the kidney and, in collaborations
with the Baban and Harris groups, whether this occurs in CKD patients and may underlie
the protective effect of NaHCO3
3. Investigating the role of mesothelial cell communication toward splenic capsular
fibrosis and anti-inflammatory signaling in the spleen. In investigating the role of the spleen in polarization of the immune system to NaHCO3, we found that simply moving the spleen during surgery was sufficient to abolish
the renal anti-inflammatory response. This observation prompted us to investigate
how anti-inflammatory signals reach the splenic parenchyma. We found that mesothelial
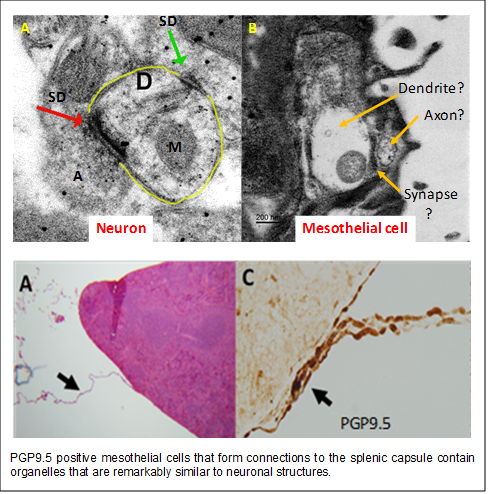
cells appear to form connections to the splenic capsule and that when these connections
are broken, mesothelial cells on the splenic capsule proliferate and hypertrophy.
This response is then followed by the development of splenic capsular fibrosis. Remarkably,
these mesothelial cells stain positive for the pan neuronal marker PGP9.5 and contain
ultrastructural organelles similar to those observed in neurons. These observations
led us to hypothesize that specialized mesothelial cells may act to communicate anti-inflammatory
signals to the spleen. O’Connor laboratory members are currently investigating whether
mesothelial cells are able to transmit signals to the splenic capsule and whether
disruption of this signaling results in splenic capsular fibrosis and immune dysregulation.
| Jenny Nguyen received the Barbara A. Horowitz and John M. Horowitz Outstanding Undergraduate Abstract Award titled, "'Assessment of temporal changes in glomerular filtration rate in male and female rats using transcutaneous FITC-sinistrin clearance." The competition was held at the APS meeting located in Long Beach, CA April 2024 and Ms. Nguyen represented the O'Connor Lab. Congratulations to Jenny Nguyen! |
Awards and Honors
2019 Hamilton Scholar in Physiology - for contributions to the Department of Physiology and Medical College of Georgia
2019 Medical College of Georgia Distinguished Faculty Award for Basic Science Teaching
2017-18 Medical College of Georgia Exemplary Teaching Award (PBL)
2018 Excellence in Teaching Award (Augusta University Graduate School)
2015 Arthur Guyton Award for Excellence in Integrative Physiology, American Physiological Society
2014 New Investigator Awardee for the Water and Electrolyte Homeostasis Section of the American Physiological Society
2013 Elected Fellow of the American Heart Association
2012 Kidney Council New Investigator Award American Heart Association, Council for High Blood Pressure Research
2012 Renal Section Research Recognition Award, American Physiological Society
Selected Professional Services
Grant Reviewer:
American Heart Association (AHA), Cardio-Renal Section
Department of Veterans Affairs, Nephrology Study section
National Institutes of Health (NIH), Re-building a kidney consortium
Associate Editor:
Molecular Medicine (2020-2023). Molecular Medicine is a peer-reviewed open access medical journal published by The Feinstein Institute for Medical Research. IF 4.096 https://molmed.biomedcentral.com/about
Comprehensive Physiology (2020-present) . Comprehensive Physiology occupies a unique niche among physiology journals and in
the APS community as a co-production of APS and Wiley Publishing Co. It is in the
top 5 journals in physiology, with an I.F of 6.604.
Editorial Boards:
American Journal of Physiology, Renal Physiology
Physiological Reports
Institutional:
Co-Program director, Physiology Graduate Program
Representative Publications
|
Ultrasound measurement of kidney volume: A sensitive indicator or severity of parenchymal injury following ischemic acute kidney injury. Gene R. Crislip, Bansari Patel, Riyaz Mohamed, Sarah C Ray, Qingqing Wei, Jingping Sun, Jennifer C. Sullivan, and Paul M. O’Connor. Am J Physiology, Renal: 2020 (In Press) |
|
Ischemic Renal Injury – Can Renal Anatomy and Associated Vascular Congestion Explain why the Medulla and not the Cortex is where the trouble starts? Ray S, Mason, J, O’Connor P.M. Seminars in Nephrology November 2019, Volume 39, Issue 6, Pages 520–529 |
|
Voltage gated proton channels modulate mitochondrial reactive oxygen species production by complex I in renal medullary thick ascending limb. Redox Biology: Bansari Patel*, Nadhezda Zheleznova*, Sarah C Ray, Jingping Sun, Allen W Cowley Jr, Paul M O’Connor. Redox Biol. 2019 Apr 16:101191. doi: 10.1016/j.redox.2019.101191 |
|
A basic solution to activate the cholinergic anti-inflammatory pathway via the mesothelium? Elinor C Mannon, Jingping Sun, Katie Wilson, Michael Brands, Patricia Martinez-Quinones, Babak Baban, Paul M O’Connor. Pharmacol Res. 2019 Mar;141:236-248 |
|
Oral NaHCO3 limits tubular cast formation independent of glomerular injury and proteinuria in Dahl salt-sensitive rats. Sarah Ray, Bansari Patel, Debra Irsik, Jingping Sun, Hiram Ocasio, Gene Crislip, Chunhua Jin, Jiankang Chen, Babak Baban, Aaron Polichnowski, Paul M. O'Connor. Clin Sci (Lond). 2018 Jun 20;132(11):1179-1197. doi: 10.1042/CS20171630. |
|
Oral NaHCO3 activates the splenic anti-inflammatory pathway; evidence vagal signals are transmitted via mesothelial cells. Sarah C Ray, Babak Baban, Matthew A Tucker, Alec J Seaton, Kyu Chul Chang, Elinor C Mannon, Jingping, Bansari Patel, Katie Wilson, Jacqueline B Musall, Hiram Ocasio, Debra Irsik, Jessica A Filosa, Jennifer C. Sullivan, Brendan Marshall, Ryan A Harris, Paul M. O’Connor. Journal of Immunology 2018 May 15;200(10):3568-3586 |
|
Hv1 acts as a sodium sensor and promotes superoxide production in medullary thick ascending limb of Dahl salt-sensitive rats. Jacob HJ, Lambert NA, O'Connor PM. Jin C, Sun J, Stilphen C.A, Smith S.M.E, Ocasio H, Bermingham B, Darji S, Guha A, Patel R, Geurts A.M, Jacob H.J, Lambert N.A, O’Connor P.M. Hypertension. 2014 Sep;64(3):541-50 |
|
Renal tubular epithelial cells regulate erythropoiesis via HIF-dependent suppression of erythropoietin production. Farsijani N.M, Liu Q, Kobayashi H, Davidoff O, Sha F, Fandrey J, Ikizler A, O’Connor P.M, Hasse V.H. J Clin Invest. 2016 Apr 1;126(4):1425-37 |
|
Medullary thick ascending limb buffer vasoconstriction of renal outer-medullary vasa recta in salt-resistant but not salt-sensitive rats. O’Connor P.M, Cowley A.W Jr. Hypertension. 2012 Oct;60(4):965-72 |
|
Increased expression of NAD(P)H oxidase subunit p67phox in the renal medulla contributes to oxidative stress and salt-sensitive hypertension. Feng D., Yang C., Geurtz A., Kurth T., Liang M., Lazar J., Mattson LD., O’Connor P.M., Cowley A.W.Jr. Cell Metabolism, 2012 (15)2:201-8 |
Related Links

- Cells that make our insides slick also calm our spleens (MedicalXpress)
- Could a Daily Dose of Baking Soda Combat Autoimmune Disease? (ReachMD)
- Drinking baking soda could be an inexpensive, safe way to combat autoimmune disease (EurekAlert!)
Men's Journal - September 2018 Vol 27, No. 9 page 111
Dr. O'Connor's Faculty Profile
LAB PERSONNEL
| Chloe Johnson Undergraduate Honors Student |
Hiram Ocasio Research Associate |
| Elinor Mannon MD/PHD student |
Sarah Ray Graduate Student |
| Jingping Sun, MD Research Associate |
 Pictured left to right: Elinor Mannon, Ellen Gillis, and Sarah Ray at the International Society of Nephrology
Conference in Melbourne. (2019)
Pictured left to right: Elinor Mannon, Ellen Gillis, and Sarah Ray at the International Society of Nephrology
Conference in Melbourne. (2019)
 O’Connor students and colleagues visiting with Drummond/Sobey laboratories at LaTrobe
University, Melbourne Australia (2019)
O’Connor students and colleagues visiting with Drummond/Sobey laboratories at LaTrobe
University, Melbourne Australia (2019)
