Therefore, we are using the recently developed model (see above) to block functional hyperemia while monitoring neuronal and astrocytic responses to sensory stimuli.

- Augusta University
- Colleges & Schools
- Medical College of Georgia
- Physiology
- Faculty | Physiology
- Philip O’Herron, PhD
Philip O’Herron, PhD
Assistant Professor

phone-office icon - (706) 721-9103
fax icon - (706) 721-7299
envelope icon poherron@augusta.edu
building icon - CA-2094
microscope icon - CA-2064
Lab Personnel

Kun Xie
- Research Associate

Dalchand Ahirwar, PhD
- Postdoctoral Fellow

Olubukola Ojo, PhD
- Postdoctoral Fellow
Jump to: Education & Past Appointments Research Interests Ongoing Research Past Research
Education & Past Appointments
Education and Training
Johns Hopkins University, Neuroscience, PhD, 2009
George Mason University, Chemistry, BA, 2002
Christendom College, Philosophy, BA, 2000
Academic Appointments
2023 | Assistant Professor, Augusta University
2018 | Research Assistant Professor, Augusta University
2017-2018 | Research Assistant Professor, Medical University of South Carolina
Research Interests
My lab’s research focuses on questions of neurovascular coupling and visual processing. Our neurovascular coupling work seeks to determine how neural activity in the cortex drives hemodynamic responses (functional hyperemia) and, in turn, how important this increased blood flow is to healthy neuronal function.
Recent Research
We recently developed a mouse model to block functional hyperemia using optogenetics. This mouse expresses the red-shifted cation channel opsin ReaChR in vascular mural cells, allowing the vessels to be constricted with light. Using two-photon activation we are able to constrict individual vessels, and using widefield single-photon activation all the vessels in the cranial window can be constricted. We also established that sensory-stimulus-evoked vasodilation can be offset by optogenetic activation of the vessels.
Optogenetic vasoconstriction
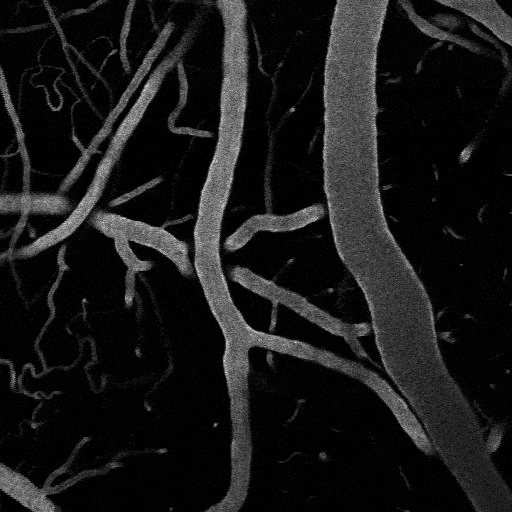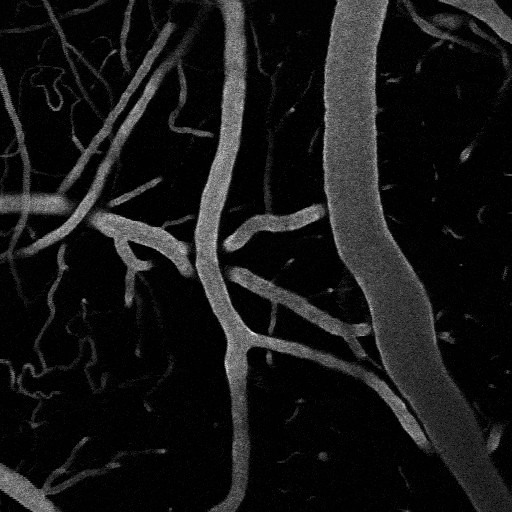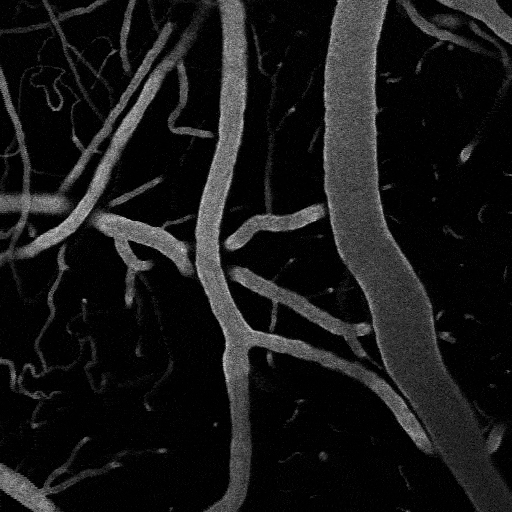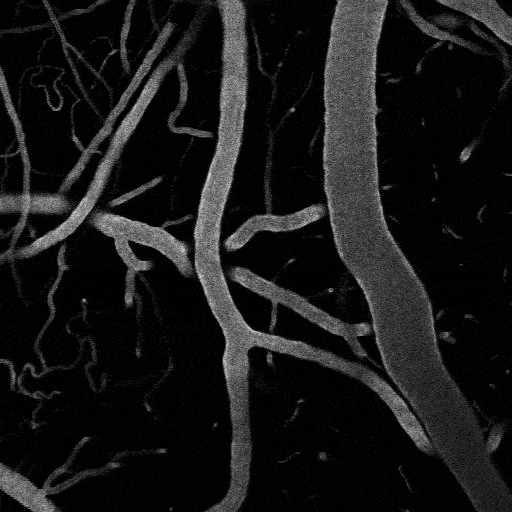
All of the arteries constrict to a 100 ms flash of the LED light (seen as a brief dimming of the image) over the cranial window. Vessels in mouse visual cortex labeled with FITC dextranand simultaneously imaged with two photon microscopy during activation.
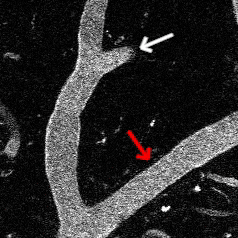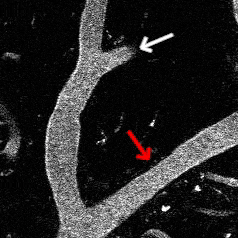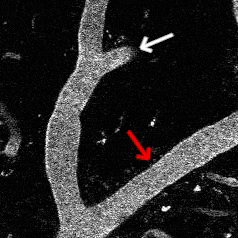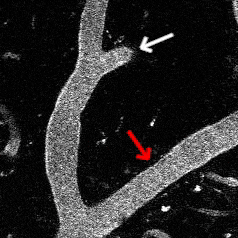
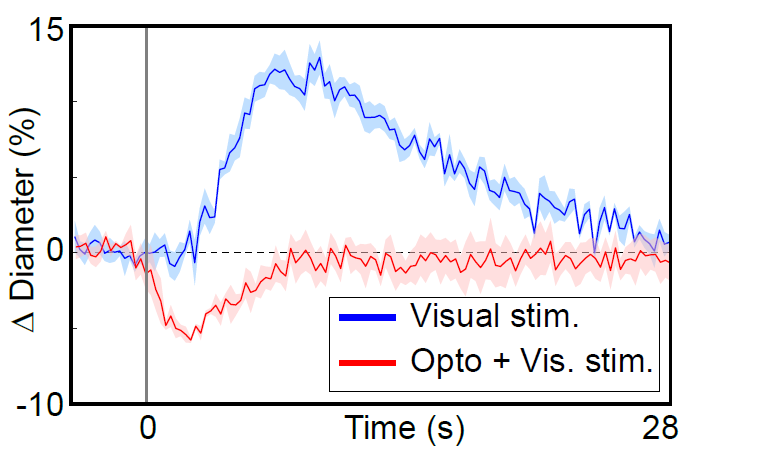
Visual stimuli activate neurons leading to dilation of vessels in the visual cortex (blue trace). When the visual stimuli are paired with pulses of light, the optogenetic evoked constriction blocks the usual functional hyperemia response (red trace).
- 3D optogenetic control of arteriole diameter in vivo
O'Herron, P. J., Hartmann, D. A., Xie, K., Kara, P. & Shih, A. Y., Sep 2022, eLife. 11, e72802.
Ongoing Research
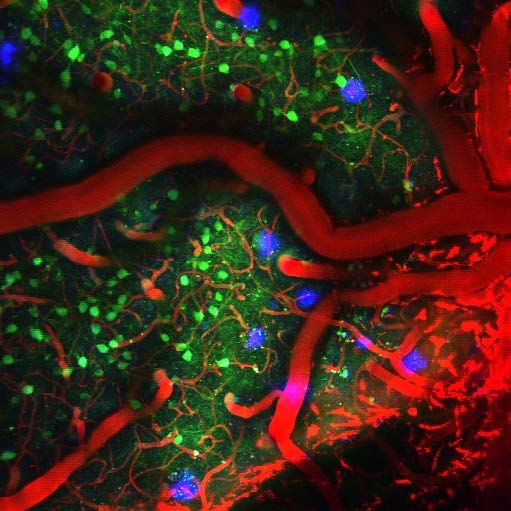
and Methoxy-X04 labelling amyloid-β plaques in mouse visual cortex.
Another ongoing project in the lab involves a collaboration with Dr. Jessica Filosa here at Augusta University. We use this same optogenetic model to constrict vessels during spontaneous states (absence of visual stimulation) to study vasculo-glia-neuronal coupling - signalling from vessels back to astrocytes and neurons. This coupling was established previously in Dr. Filosa’s lab in brain slices (Kim et al. J Neuroscience 2016). We are now seeking to determine if vasoconstriction in vivo can directly alter astrocytic and neural activity patterns.
We are currently investigating the role functional hyperemia plays in healthy neural function. It has been known for more than a century than brain activity causes increases in local blood flow, and the widely held assumption is that this is necessary for providing increases in oxygen and glucose to support the increase in neural activity. However, this hypothesis has never been directly tested and there are reasons to believe that this may not be the full explanation.
- We use two-photon imaging to determine if response strength and selectivity are altered by the loss of functional hyperemia. Electrophysiological recordings are used to determine if action potential timing and network synchrony are also affected.
- Another component of our project - which is a collaboration with Dr. Felipe Barros from the CECS in Valdivia Chile - is to determine if metabolic processing in neurons and astrocytes adapts to the reduction in functional hyperemia to enable sustained neural activity in the face of reduced blood flow. We use fluorescent imaging and other measures of metabolic substrates to address this question.
- We are also examining if functional hyperemia is more critical in aging and disease. We use a mouse model of Alzheimer’s disease to study the interaction of neural and vascular dysfunction with disease pathology to understand the role blood flow plays in the development of the disease.
Past Research
Past Research
My introduction to neurovascular coupling came during my post-doctoral fellowship.
There I studied the correspondence between regions of active neural tissue and regions
of increased blood flow. We found that both neuronal spiking and synaptic activity
were more anatomically localized than the regional increase in blood flow. This work
will help to interpret hemodynamic imaging studies which use vascular signals as surrogates
for neural activity.
- Neural correlates of single-vessel haemodynamic responses in vivo. O'Herron P, Chhatbar
PY, Levy M, Shen Z, Schramm AE, Lu Z, Kara P (2016) Nature 534:378-382
- Spotlighted in Neuron: Denfield George H, Fahey Paul G, Reimer J, Tolias Andreas S (2016) Investigating the Limits of Neurovascular Coupling. Neuron 91:954-956
- Targeted Labeling of Neurons in a Specific Functional Micro-domain of the Neocortex by Combining Intrinsic Signal and Two-photon Imaging. O’Herron P., Shen Z., Lu, Z., Schramm A., Levy M., & Kara P. (2012). Journal of Visualized Experiments (70): e50025
- An artery-specific fluorescent dye for studying neurovascular coupling in vivo. Shen, Z.M., Lu, Z.Y., Chhatbar, P.Y., O’Herron, P., Kara, P. (2012). Nature Methods 9, 273-276.
Visual Processing Research
My PhD work was on visual processing in primate visual cortex. I studied figure-ground signals using electrophysiological recordings.
- Short-term memory for figure-ground organization in the visual cortex. O'Herron, P., and von der Heydt, R. (2009). Neuron 61, 801-809.
- Representation of object continuity in the visual cortex. O'Herron, P., and von der Heydt, R. (2011). Journal of Vision 11(2): 12.
- Remapping of border ownership in the visual cortex. O'Herron, P., and von der Heydt,
R. (2013). Journal of
Neuroscience 33(5): 1964-1974
More recently, I have also studied how neuronal response properties vary with depth in the upper layers of mouse primary visual cortex
- An Unexpected Dependence of Cortical Depth in Shaping Neural Responsiveness and Selectivity in Mouse Visual Cortex. O’Herron P., Levy M., Woodward J. J. & Kara P. (2020) eNeuro, ENEURO.0497-0419.2020
