Sergei Kirov, PhD
Professor,
Dept. of Neuroscience & Regenerative Medicine
Mailing address:
Dept. of Neuroscience & Regenerative Medicine
1120 15th Street, Rm. CA4008
Medical College of Georgia at Augusta University
Augusta, GA 30912
Phone: 706-721-6051
E-mail: skirov@augusta.edu
Education:
College/University:
1982-1987 St.-Petersburg (former Leningrad) State University, St.-Petersburg,
Russia; M.S. in Biophysics
Graduate and Professional:
1988-1991 St.-Petersburg State University & Pavlov Institute of Physiology,
St.-Petersburg, Russia; Ph.D. in Biophysics
Post-Doctoral Training:
1992-1995 Postdoctoral Fellow, NIH, National Institute on Aging, Gerontology
Research Center, Baltimore, Maryland
1995-1997 Senior Postdoctoral Fellow, Dept. of Physiology, University of Maryland, School of Medicine, Baltimore, Maryland
1997-1999 Research Associate, Division of Neuroscience, Harvard Medical School, Children’s Hospital, Boston, Massachusetts
Research interests:
My background is in Neuroscience and Biophysics, with specific training in imaging, electrophysiology, and electron microscopy. My Multiphoton Imaging Laboratory studies mechanisms for neural circuit dysfunction in preclinical models of stroke and TBI. We are integrating a variety of classic and state of the art technologies; viral expression, mouse genetics, in vivo 2-photon microscopy coupled with electrophysiology, laser speckle imaging, and functional intrinsic optical signal imaging, as well as ultrastructural studies with serial section transmission electron microscopy. Such array of sophisticated methods provides a dramatic window several hundred microns deep into the cortex to identify and track, in real-time, responses to neurological insult in defined circuits and cells. Our goal is to attenuate mechanisms that initiate and propagate local injury and amplify responses that promote recovery.
Current projects:
Cortical Spreading Depolarization: Emerging pathophysiologic mechanism in the acutely
injured brain
Recent clinical data indicate that repeated waves of spreading depolarizations are
involved in the mechanism of injury in patients with stroke and brain trauma. We are
aiming to understand the causality between changes in neurons, astrocytes, synapses,
blood flow, and spreading depolarization. The research has obvious clinical implication
by pointing to spreading depolarization as an important mechanistic endpoint in treating
these neurological disorders.
Molecular mechanisms of cytotoxic edema
Normal brain function invariably depends on water homeostasis. Disturbance of this
homeostasis during stroke and TBI leads to a life-threatening state of brain edema.
We are aiming to identify the exact molecular pathways of water influx into neurons
at the onset of cytotoxic edema to explore strategies for rescue neurons from swelling.
Injury-induced mitochondrial dynamics
Abnormal mitochondrial dynamics and fragmentation of the mitochondrial network into
small spherical structures are considered hallmarks of mitochondrial injury. Excessive
fragmentation facilitates apoptosis. Until recently live imaging studies of mitochondria dynamics in vivo were not conducted in the intact brain. Using 2-photon microscopy, we were able to
overcome this critical barrier and, for the first time, quantified dendritic mitochondrial
fragmentation in real-time in vivo immediately after insult and throughout three weeks in response to injury. We are
conducting studies to determine how mitochondrial structural rearrangements affect
neuronal survival and if therapeutic approaches targeting this organelle can improve
neural pathological outcomes.
A protective role of astrocytes against synaptic injury in stroke and brain trauma
Disruption of synaptic networks after stroke or TBI involves the impairment of astrocytes
alongside neuronal dysfunction. My research group was among the first to image astroglial
responses to ischemia in vivo using 2-photon microscopy. Our studies revealed critical new information about dynamic
astroglial responses to interruptions in blood flow and osmotic stress. We are conducting
studies on how impaired astroglial metabolism aggravates neuronal injury during stroke
and how functioning astrocytes facilitate neuronal recovery after stroke.
Two-photon imaging in the neocortex of a living mouse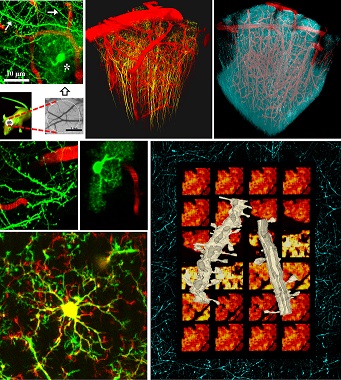
Top row: Schematic of intravital imaging showing dendrites (arrows), astrocyte (asterisk)
and capillaries (red). Next two images are the 3D reconstruction of dendrites of pyramidal
neurons (yellow), dendritic mitochondria (cyan) and blood vessels (red).
Middle row: Intravital images of dendrites with dendritic spines and astrocyte.
Bottom left: Overlay showing two merged images of microglial cell obtained at a 20 min interval
revealing frequent extension (green) and retraction (red) of microglial processes.
Bottom right: Dendritic segments from sham-operated (left, with tubular mitochondria)
and ischemic (right, with fragmented mitochondria) mice reconstructed in three dimensions
from serial electron microscopic images superimposed onto a montage showing changes
in neuronal mitochondria morphology after stroke. The frame is a two-photon image
of CFP-labeled neuronal mitochondria from a sham-operated mouse.
Selected publications:
- Kirov SA, Fomitcheva IV, Sword J. (2020) Rapid Neuronal Ultrastructure Disruption and Recovery during Spreading Depolarization-Induced Cytotoxic Edema. Cereb Cortex. 2020 Sep 3;30(10):5517-5531. doi: 10.1093/cercor/bhaa134.
- Kislin M, Sword J, Fomitcheva IV, Croom D, Pryazhnikov E, Lihavainen E, Toptunov D, Rauvala H, Ribeiro AS, Khiroug L, Kirov SA. (2017) Reversible disruption of neuronal mitochondria by ischemic and traumatic injury revealed by quantitative two-photon imaging in the neocortex of anesthetized mice. J. Neurosci. 37(2), 333-348. PMID: 28077713. ƗFeatured Article in the “This Week in The Journal”: http://www.jneurosci.org/content/37/2/i
- Sword J, Croom D, Wang PL, Thompson RJ, Kirov SA. (2017) Neuronal pannexin-1 channels are not molecular routes of water influx during spreading depolarization-induced dendritic beading. J. Cereb. Blood. Flow Metab., 37(5):1626-1633. PMID: 26994044.
- Steffensen AB, Sword J, Croom D, Kirov SA, MacAulay N. (2015) Chloride cotransporters as a molecular mechanism underlying spreading depolarization-induced dendritic beading. J. Neurosci. 35(35), 12172-12187. PMID: 26338328. ƗFeatured Article in the “This Week in The Journal”: http://www.jneurosci.org/content/35/35/i ƗƗFeatured Article on Neuronline: http://neuronline.sfn.org/Articles/Scientific-Research/2017/Cotransporters-in-Spreading-Depolarization-Induced-Neuronal-Swelling
- Sword J, Masuda T, Croom D, Kirov SA. (2013) Evolution of neuronal and astroglial disruption in the peri-contusional cortex of mice revealed by in vivo two-photon imaging. BRAIN, 136(Pt 5): 1446-1461. PMID: 23466395.
- Risher WC, Croom D, Kirov SA. (2012) Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. GLIA, 60(11): 1709-1720. PMID: 2821441.
- Masuda T, Croom D, Hida H, Kirov SA. (2011) Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. GLIA, 59(11): 1744-1753. PMID: 21800362. **Cover illustration from this article.
- Risher WC, Ard D, Yuan J, Kirov SA. (2010) Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J.Neurosci, 30(29), 9859-9868. PMID: 20660268.
- Witcher MR, Park YD, Lee MR, Sharma S, Harris KM, Kirov SA (2010) Three-dimensional relationships between perisynaptic astroglia and human hippocampal synapses. GLIA, 58(5), 572-587. PMID:19908288. **Cover illustration from this article.
- Risher WC, Andrew RD, Kirov SA. (2009) Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. GLIA, 57(2), 207-221. PMID: 18720409. **Cover illustration from this article.
- Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. (2007) Physiological evidence that pyramidal neurons lack functional water channels. Cereb. Cortex. 17(4), 787-802. PMID: 16723408.
https://www.ncbi.nlm.nih.gov/myncbi/sergei.kirov.1/bibliography/public/
2-Photon Microscopy Core: https://www.augusta.edu/mcg/dnrm/microscopes.php