Dr. Eric Vitriol

Eric Vitriol, PhD
Associate Professor
Director, Neuroscience PhD Program
Dr. Eric Vitriol received his B.S. and Ph.D. from the University of North Carolina at Chapel Hill, under the mentorship of Ken Jacobson & Klaus Hahn. In 2009, he began to study the neuronal cytoskeleton as a postdoctoral fellow in the lab of James Zheng at Emory University. Dr. Vitriol joined Augusta University in 2021 as an Associate Professor, where he leads a research lab using advanced imaging & biochemical techniques to understand the cellular mechanisms of neurodegenerative disease. He has a strong commitment to mentoring students & fostering interdisciplinary collaborations to help advance the understanding of cellular neurobiology.
Contact Us
Eric Vitriol Lab
1462 Laney Walker Blvd.
CA 3012
706-721-1175
Lab - 706-721-1176
Research Interests
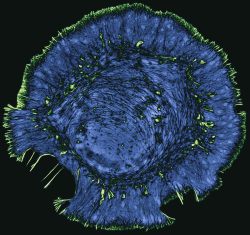
My lab studies the role of the actin cytoskeleton in cell motility, neural development, and in neurodegenerative diseases such as Alzheimer’s and amyotrophic lateral sclerosis (ALS). We use live-cell and super-resolution imaging to understand how the dynamic regulation of actin contributes to the normal function of healthy cells, and how defects in actin can cause toxicity and cell death. We also benefit greatly from collaborations with computational groups, who help us build mathematical models and develop new methods of image analysis so that we can get the most out of our microscopy data. We purposely seek out unconventional questions that will broaden our understanding of how the cytoskeleton controls cell physiology. Our ultimate goal is to integrate imaging-based cell biology with translational science to understand how cell-based processes contribute to human disease.
Open Positions
We celebrate the strengths of people from diverse backgrounds and encourage those underrepresented in STEM to apply. If you are pursuing a research opportunity, please include a current CV and a brief statement about why the Vitriol lab is of interest in the initial email.
Graduate students
We welcome potential graduate students to apply for a rotation. Students are accepted through the Graduate Program in Neuroscience.
Undergraduate students
We accept undergraduate students who are interested in doing research for course credit through CURS. Must be willing to commit at least 8 hours per week in the lab. No previous experience needed- we will teach you everything that you need to know!
Current Projects
The role of PFN1 in organelle homeostasis
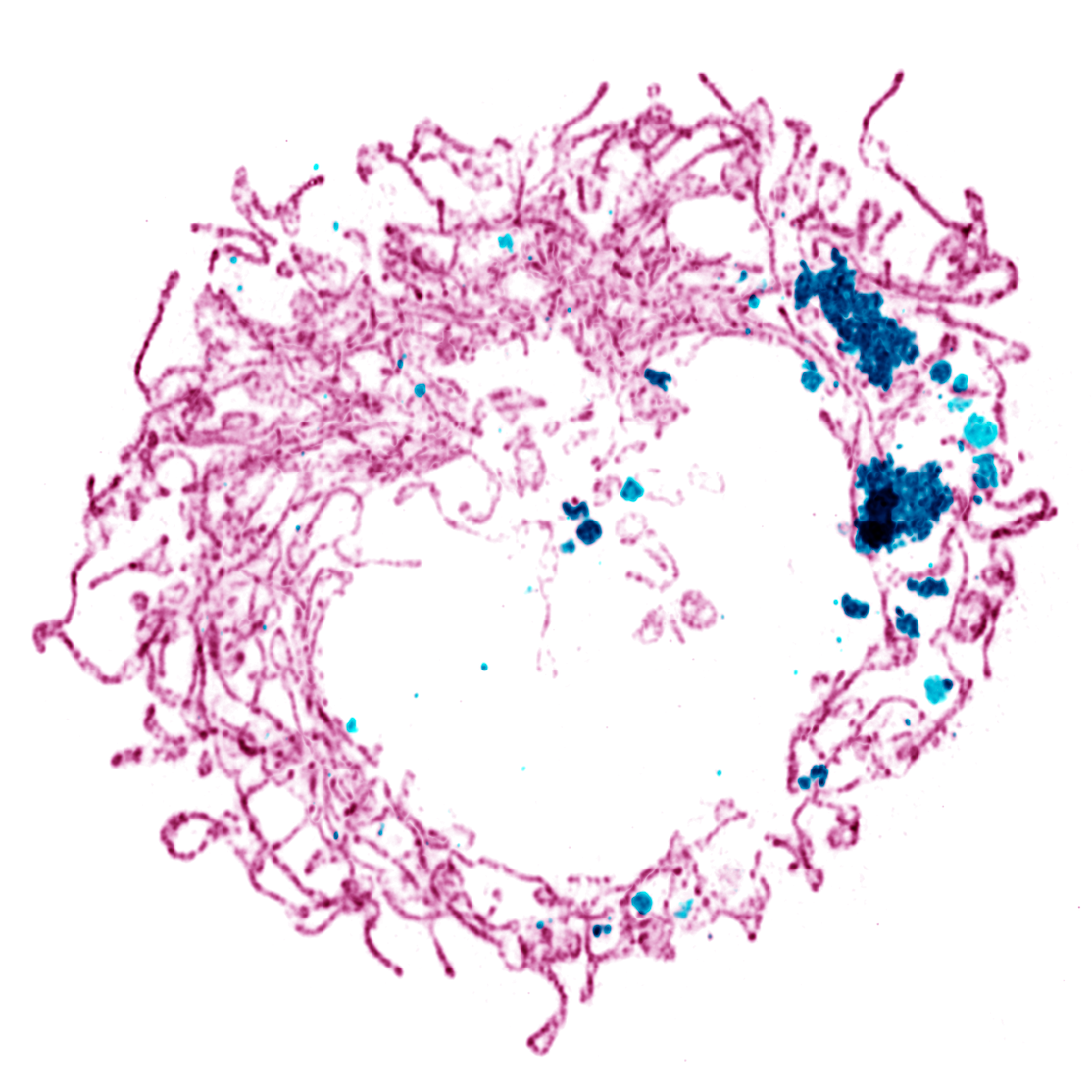
Profilin 1 (PFN1) is a key player in cellular structure and function, serving as the predominant actin monomer-binding protein in mammalian cells. Recent studies into the pathogenic mechanisms caused by PFN1 mutations have greatly expanded our understanding of its crucial role in cellular physiology. For example, we have discovered that PFN1 is essential for maintaining mitochondrial integrity, with the loss of PFN1 expression leading to the accumulation of depolarized, dysmorphic mitochondria. Surprisingly, this disruption in mitochondrial homeostasis is believed to result from a specific and novel role of PFN1 within the mitochondrial matrix. Additionally, we have identified critical functions for PFN1 in regulating the clearance of damaged organelles and the endoplasmic reticulum. However, many questions about PFN1’s role in these processes remain. We are now employing novel methods to spatiotemporally manipulate profilin expression and function, allowing us to distinguish between cause and effect and to assess both the direct actions of PFN1 and potential indirect effects arising from cytoplasmic actin assembly. These techniques are combined with super-resolution microscopy and spatial proteomics to elucidate the specific, mechanistic roles of profilin in organelle homeostasis.
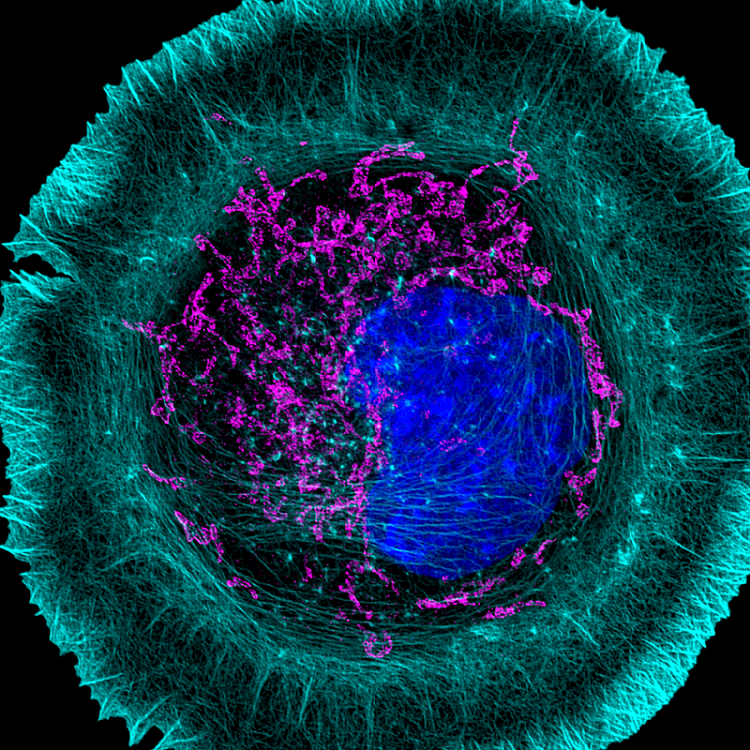

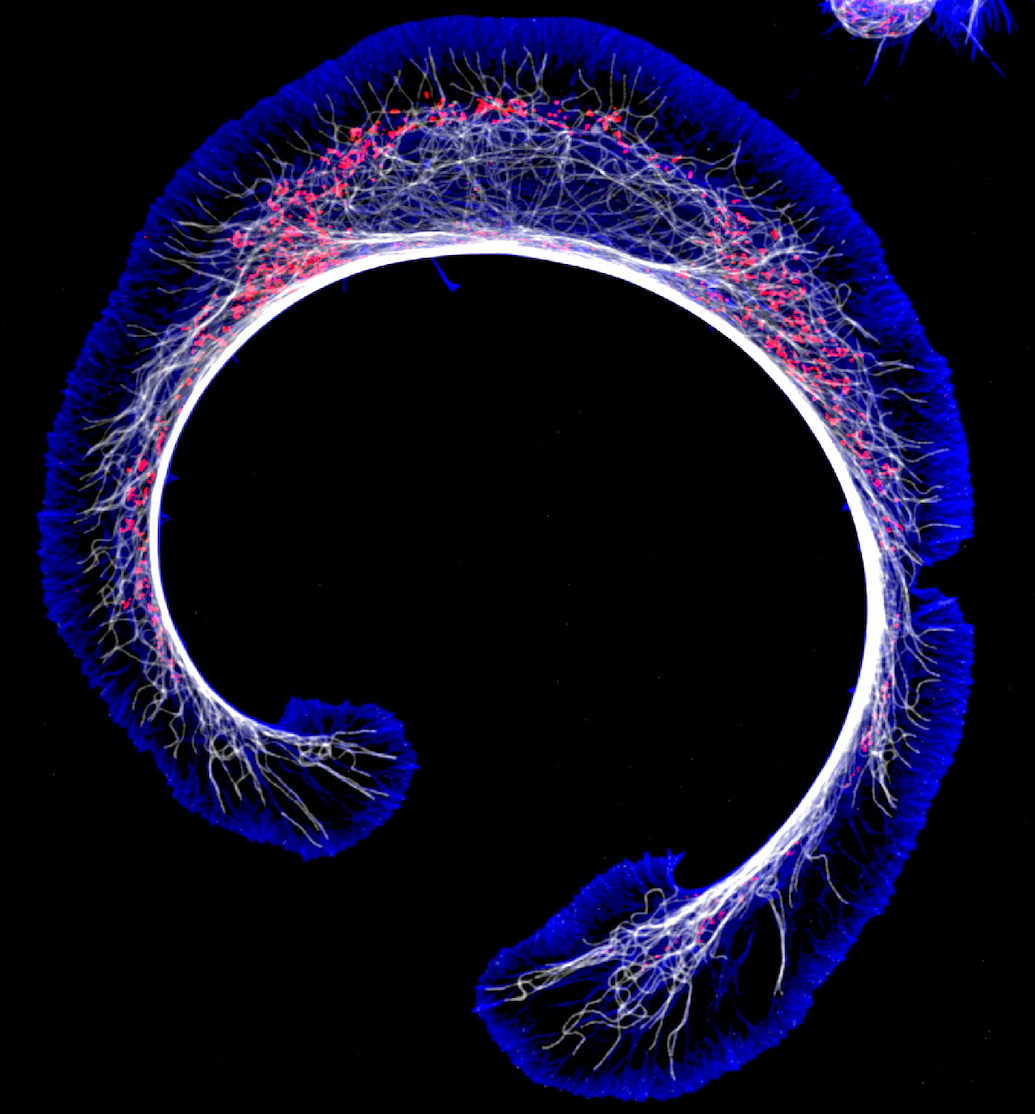
Dysregulation of actin in neurodegenerative disease
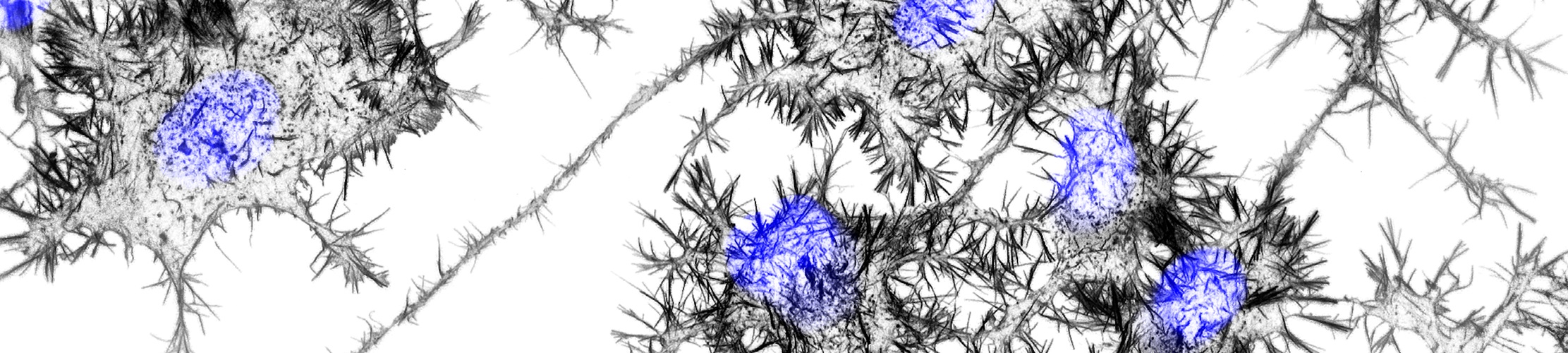
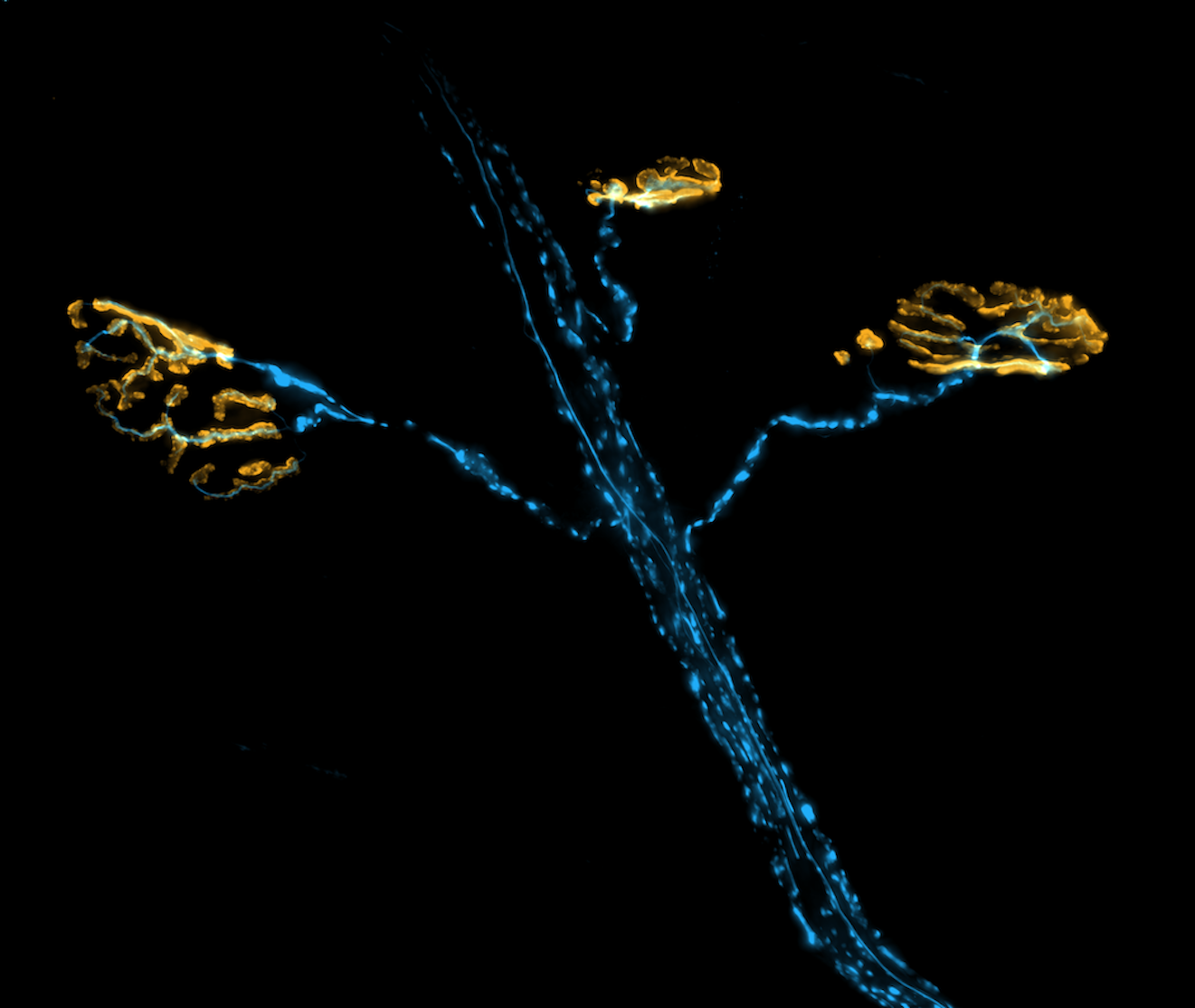
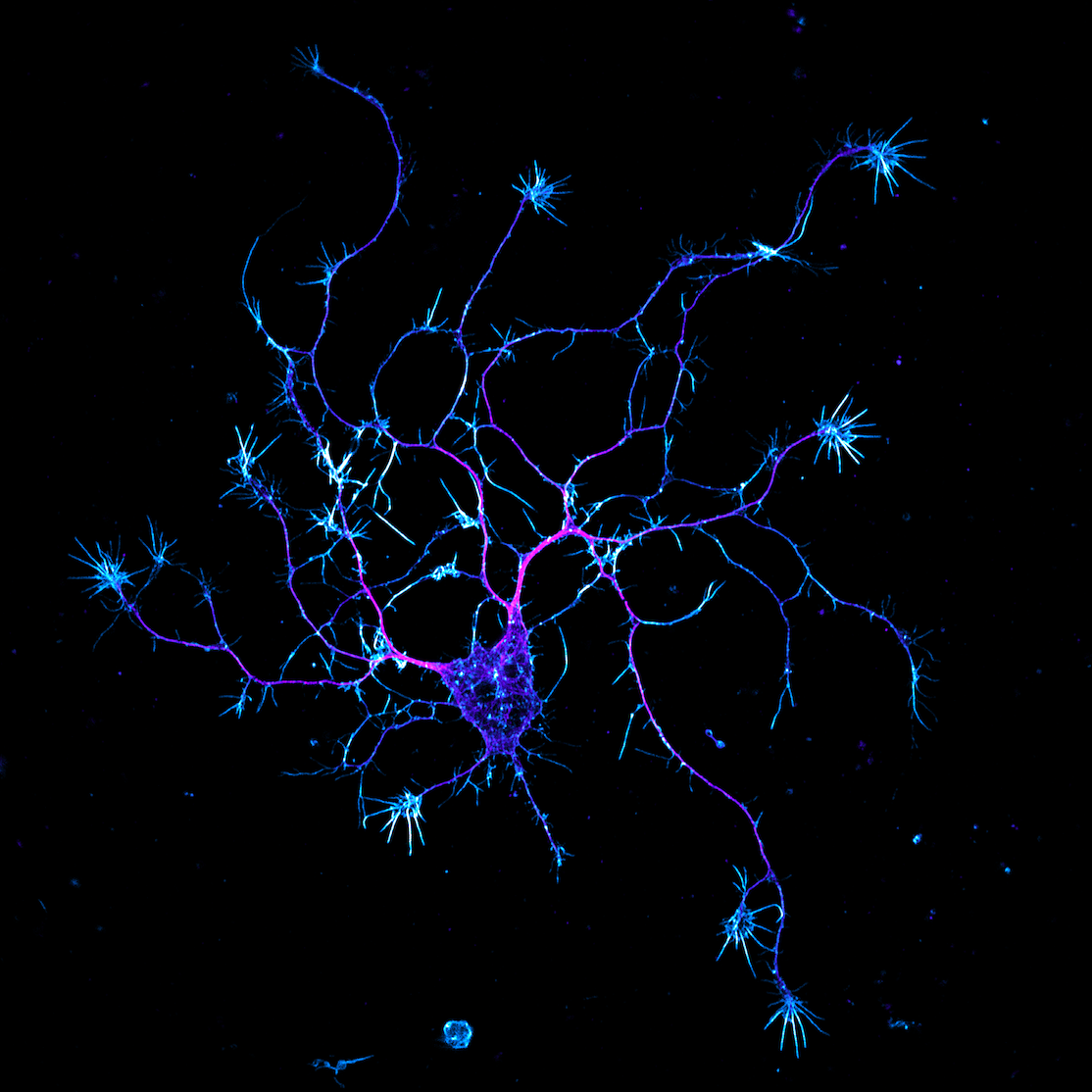
Another of my lab’s interests is understanding the connection between the actin cytoskeleton and neurodegenerative diseases like amyotrophic lateral sclerosis (ALS). Actin was first implicated in ALS when it was discovered that mutations in PFN1, a gene that encodes an actin-binding protein, cause a hereditary form of the disease. We currently have projects in the lab determining how ALS-linked mutants disrupt actin in ways that could contribute to neurodegeneration. We have also begun to investigate how dysregulation of actin in neuronal processes contributes to Alzheimer’s and Parkinson’s disease. We use neurons differentiated from human stem cells as a translational model for neurodegeneration in the lab. We then apply spatial proteomics, expansion microscopy, and super-resolution imaging to identify the early molecular events that cause toxicity and cell death.
Selected Publications
Lindamood HL, Liu TM, Read TA, Vitriol EA. (2024) Using ALS to understand profilin 1’s diverse roles in cellular physiology. Cytoskeleton. Online ahead of print. Link
Read TA, Cisterna BA, Skruber K, Ahmadieh S, Liu TM, Vitriol JA, Shi Y, Black JB, Butler MT, Lindamood HL, Lefebvre AEYT, Cherezova A, Ilatovskaya DV, Bear JE, Weintraub NL, Vitriol EA. (2024) The actin binding protein profilin 1 localizes inside mitochondria and is critical for their function. EMBO Reports, 25(8):3240-3262. Link
Cisterna BA, Skruber K, Jane ML, Camesi CI, Nguyen ID, Liu TM, Warp PV, Black JB, Butler MT, Bear JE, Mor DE, Read TA, Vitriol EA. (2024). Prolonged depletion of profilin 1 or F-actin causes an adaptive response in microtubules. Journal of Cell Biology, 223 (7): e202309097. Link
Vitriol EA, Quintanilla MA, Tidei JJ, Troughton LD, Cody A, Cisterna BA, Jane ML, Oakes PW, Beach JR. (2023) Non-muscle myosin 2 filaments are processive in cells. Biophysical Journal, 122 (18): 3678-3689. Link
Edwards P, Skruber K, Milićević N, Heidings JB, Read TA, Bubenik P, Vitriol EA. (2021) TDAExplore: quantitative analysis of fluorescence microscopy images through topology-based machine learning. Patterns, 2(11): 100367. Link
Skruber K, Read TA, Vitriol EA. (2021) Delivering Defined Amounts of Purified Protein with High Precision into Living Cells. STAR Protocols, 2(1): 100272 Link
Skruber K, Warp PV, Shklyarov R, Thomas JD, Swanson MS, Henty-Ridilla JL, Read TA, Vitriol EA. (2020) Arp2/3 and Mena/VASP Require Profilin 1 for Actin Network Assembly at the Leading Edge. Current Biology, 30(14):2651-2664. Link
Osking Z, Ayers JI, Hildebrandt R, Skruber K, Eukovich AR, Brown H, Ryu D, Golde TE, Borchelt DR, Read TA, Vitriol EA. (2019) ALS-linked SOD1 Mutants Enhance Outgrowth and Branching in Adult Motor Neurons. iScience 11:294-304. Link