Research in the LeMosy Lab
Project One: How does a matrix protein regulate both formation of the craniofacial skeleton in zebrafish, and left-right patterning of its heart?
Our current major project centers on studies in zebrafish of conserved ECM proteins implicated in pediatric skeletal and kidney disease and in suppressing tumor metastasis. This project offers several opportunities for trainees to develop independent and/or collaborative projects.
Knocking the zebrafish protein out (CRISPR/Cas9 mutant) or reducing its expression (morpholino oligonucleotides, MOs) results in similar defects, of which just two will be described here. Characterized so far just for the MO method, there are dose-dependent reductions in the craniofacial cartilages at 5 days of development, associated with earlier decreased dlx2a-expressing neural crest cells in the pharyngeal pouches by 1 day of development.
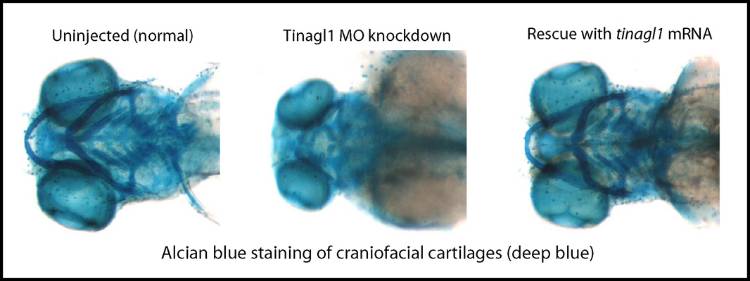
We are following up hypotheses that neural crest cell proliferation or migration is affected, and that these defects arise via reduced Wnt signaling. A paper describing data leading up to these hypotheses is in press: Neiswender, H., Navarre, S., Kozlowski, D. J., and LeMosy, E. K. (2016) Early craniofacial defects in zebrafish having reduced function of a Wnt-interacting extracellular matrix protein, Tinagl1. Complementing preliminary data from Dheeraj Ravilla’s CURS project suggests that mutating a prospective Wnt-binding site in Tinagl1 can result in dominant negative effects on zebrafish development.
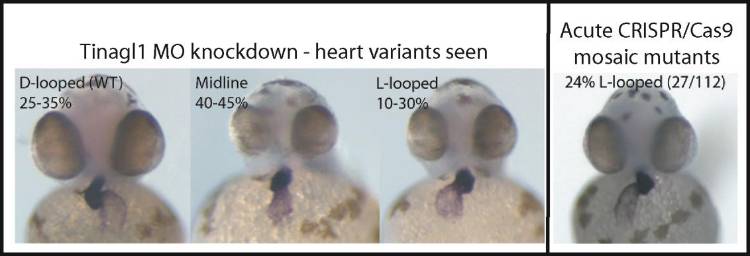
Interestingly, in both mutant and MO knockdown, we also see randomization of heart looping in the early embryo. This phenotype is a reflection of impaired establishment of the left-right (L/R) axis, not only in zebrafish but in mammals, where a classically described disease, Kartagener’s syndrome (aka primary ciliary dyskinesia), as well as numerous other genetic defects can result in failure to properly specify the L/R position of abdominal organs, lungs, and heart. We are working to identify the specific pathways that are affected by loss of this protein, and to understand how the heart looping defect relates to other phenotypes of this mutant.
Project Two: How do extracellular matrix carbohydrates and spatial cues regulate the activity state and localization of signaling molecules during fruit fly embryonic patterning?
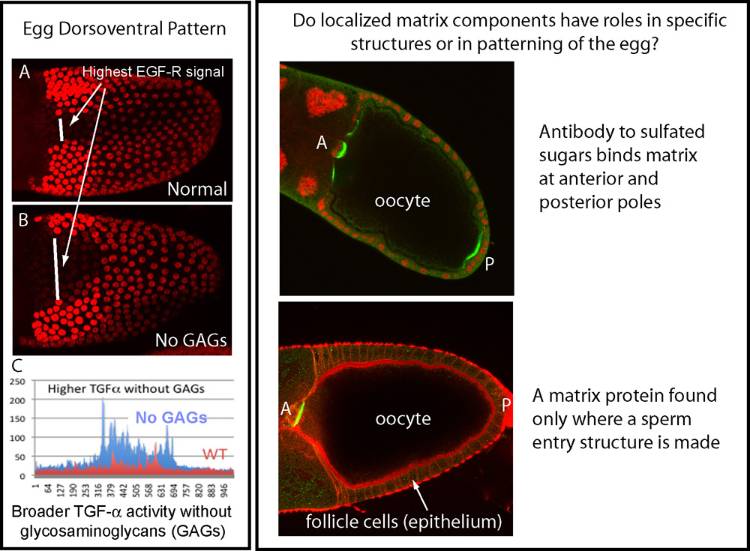
Signaling molecules similar to EGF and NGF sequentially determine dorsoventral polarity of the fruit fly oocyte and embryo. Much of our published work involves proteases that regulate activation of the NGF-related ligand in the embryo, but we have more recently gained biochemical and genetic evidence for roles of two classes of charged sugars, including GAGs (glycosaminoglycans), in spatial control of these ligands. This work may have implications for regulation of related signals important in cancer and nervous system. This project has benefited from collaborations with colleagues at the Complex Carbohydrate Research Center in Athens, GA, as well as with a laboratory at Scripps Research Institute in La Jolla, CA. For a motivated student, this project may offer experience with glycobiology and biochemistry, in addition to genetics and developmental biology.